


On December 1, 2020, the Centers for Medicare & Medicaid Services (CMS) introduced revisions to the ICD-10-PCS codes, while the most recent batch of CPT® codes was unveiled on January 25, 2021. The American Medical Association (AMA) has been consistently updating CPT codes in response to the release of vaccines, while CMS has been progressively publishing HCPCS codes related to vaccine products. These updates occur frequently, underscoring the importance of timely and thorough information review.
For the immunization administration, a singular diagnosis code exists in ICD-10-CM, denoted as Z23. This code is applicable when a patient undergoes COVID-19 vaccination. Recently, the ICD-10-PCS codes for vaccine administration have been made available, encompassing the following:
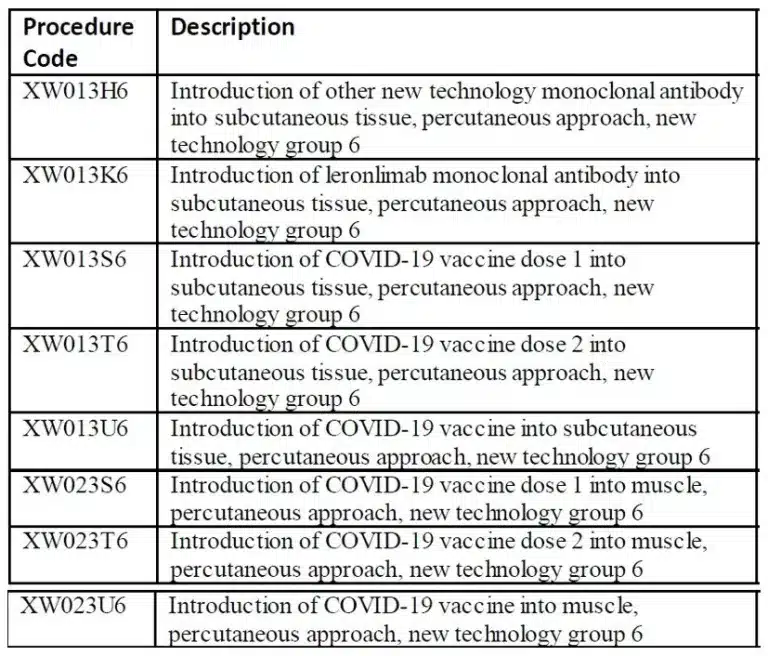
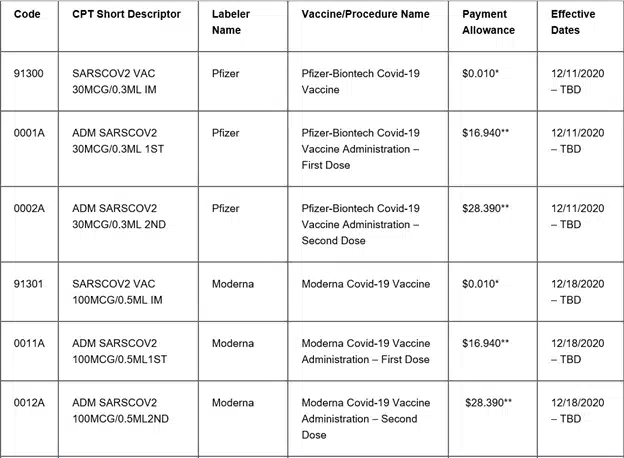
In outpatient coding, the range of codes for vaccine administration and products is expanding. The latest update from CMS encompasses codes for both the Moderna and Pfizer vaccines, along with the corresponding administration codes. This recent compilation comprises not only the existing vaccines but also those that are currently in the developmental stage. It’s essential to bear in mind that distinct codes are assigned to the administration of each individual vaccine as well as its corresponding product. Refer to the provided list below for a comprehensive overview of the current vaccines and their corresponding administration codes:
This list includes proposed vaccines and their associated administration:
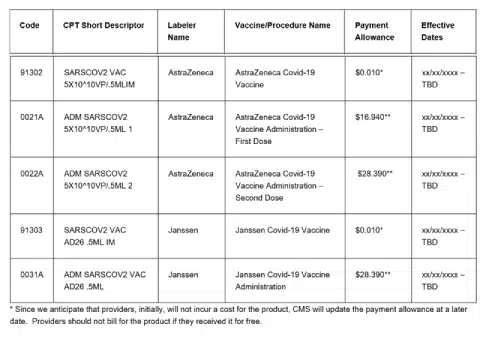
At present, the government is shouldering the expenses associated with the vaccine itself; however, organizations are permitted to impose charges for the administration of the vaccine. If you haven’t yet engaged in a dialogue regarding your facility’s specific coding protocols concerning data collection for COVID, now is the opportune moment to initiate that conversation. Notably, the AMA is actively broadening the scope of CPT codes, and CMS is continually introducing new vaccine product codes as novel vaccines emerge. Have you identified the specific departments that necessitate the COVID-19 vaccine administration details? Furthermore, has the administration process been pre-established in the chargemaster, or does it require allocation by the Health Information Management (HIM) department? The responses to these inquiries will undoubtedly influence the development of coding guidelines within your facility.